SOD1 Cu/Zn
– 4.900,00€
Human, recombinant
Full length, UniProtKB accession P00441
MW = 32600 Da (dimer)
EC # 1.15.1.1.
CAT # G06SD001
| Catalog n. | Labeling | Qty | Price |
|---|---|---|---|
| 170,00€ | |||
| 520,00€ | |||
| 840,00€ | |||
| 2.600,00€ | |||
| 4.400,00€ | |||
| 195,00€ | |||
| 600,00€ | |||
| 970,00€ | |||
| 3.000,00€ | |||
| 4.900,00€ | |||
| Request a quote | |||
| Request a quote | |||
| Request a quote | |||
| Request a quote | |||
| Request a quote | |||
| VAT not included | |||
For any special request or bulk quantities Click Here
Description
Description
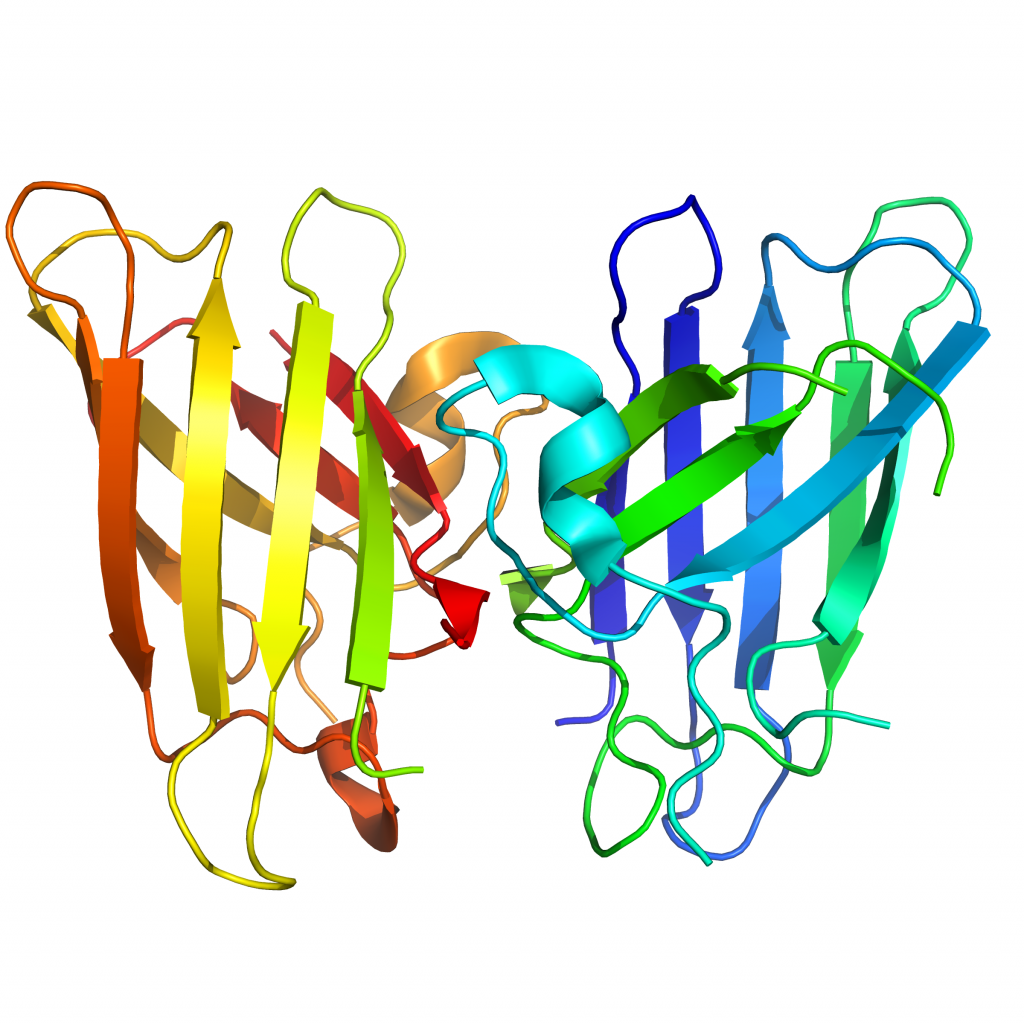
MW = 32.6 kDa (dimer) calculated, 32.9 kDa (metallated dimer). Human WT-SOD1 cloned from Human cDNA, expressed in E. coli. The protein consists of the full length human Cu, Zn SOD1 S-S (residues 1-154, UniProtKB accession P00441). Four additional residues, GSFT, are present at the N-term of the monomers.
MW = 32.6 kDa (dimer) calculated, 32.9 kDa (metallated dimer). Human WT-SOD1 cloned from Human cDNA, expressed in E. coli. The protein consists of the full length human Cu, Zn SOD1 S-S (residues 1-154, UniProtKB accession P00441). Four additional residues, GSFT, are present at the N-term of the monomers.
Sequence
10 20 30 40 50
GSFT-MATKAVCVLK GDGPVQGIIN FEQKESNGPV KVWGSIKGLT EGLHGFHVHE
60 70 80 90 100
FGDNTAGCTS AGPHFNPLSR KHGGPKDEER HVGDLGNVTA DKDGVADVSI
110 120 130 140 150
EDSVISLSGD HCIIGRTLVV HEKADDLGKG GNEESTKTGN AGSRLACGVI
GIAQ
Available mutant
- Ala89Val
- Asp90Ala *
- Glu100Gly
- Gly93Ala *
- Gly93Arg
- Gly93Asp *
- Ile113Phe *
- Ile113Thr *
- Ile35Thr
- Leu144Phe *
- Leu148Ile
- Leu67Val
- Thr54Arg *
- Val87Met *
- Val97Met *
*Amyotrophic lateral sclerosis (ALS) related.
Purity
> 95% by SDS-PAGE. The protein is observed, in denaturing conditions, as single band (monomer) migrating at a molecular weight between 14.4 and 18.4 kDa. The Cu,Zn SOD1 S-S is stable as dimer. The metallation state is verified by NMR and/or mass analysis.
> 95% by SDS-PAGE. The protein is observed, in denaturing conditions, as single band (monomer) migrating at a molecular weight between 14.4 and 18.4 kDa. The Cu,Zn SOD1 S-S is stable as dimer. The metallation state is verified by NMR and/or mass analysis.
Supplied as
1.0 mg/mL solution in sodium phosphate buffer 50 mM, pH 7.0. The concentration is calculated by the analysis of the absorbance at 265 nm (ε265 = 15900 M-1cm-1 calculated).
1.0 mg/mL solution in sodium phosphate buffer 50 mM, pH 7.0. The concentration is calculated by the analysis of the absorbance at 265 nm (ε265 = 15900 M-1cm-1 calculated).
Storage
-20°C. The protein is stable at 4°C for at least 2 weeks and at 25°C for several hours. After initial defrost, aliquot the product into individual tubes and refreeze at -20°C. Avoid repeated freeze/thaw cycles.
-20°C. The protein is stable at 4°C for at least 2 weeks and at 25°C for several hours. After initial defrost, aliquot the product into individual tubes and refreeze at -20°C. Avoid repeated freeze/thaw cycles.
References
Banci, L. et al. PLoS One 3 (2), 1-8 (2008).
Banci, L. et al. Proc. Natl. Acad. Sci. 104 (27), 11263-11267 (2007).
Banci, L. et al. J. Biol. Chem. 281 (4), 2333-2337 (2006).
Arnesano, F. et al. J. Biol. Chem. 279 (46), 47998-48003 (2004).
Banci, L. et al. PLoS One 3 (2), 1-8 (2008).
Banci, L. et al. Proc. Natl. Acad. Sci. 104 (27), 11263-11267 (2007).
Banci, L. et al. J. Biol. Chem. 281 (4), 2333-2337 (2006).
Arnesano, F. et al. J. Biol. Chem. 279 (46), 47998-48003 (2004).