Description
Description
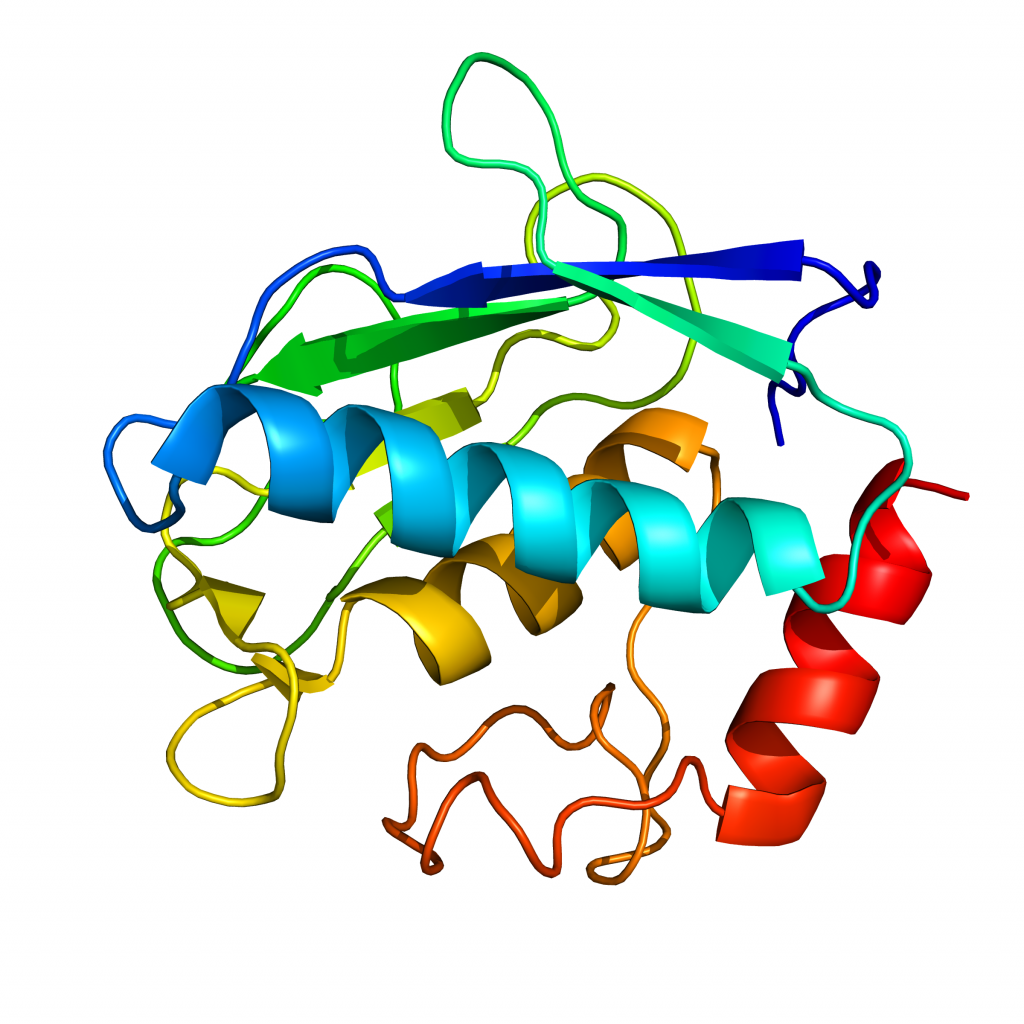
MW = 17.6 kDa calculated. Recombinant Matrix Metalloproteinase-12 (MMP-12, Metalloelastase, Macrophage elastase) cloned from human cDNA, expressed in E. coli. The enzyme consists of the wild type catalytic domain of human MMP-12 (residues 106-263, UniProtKB accession P39900).
Sequence
110 120 130 140 150
M-GPVWR KHYITYRINN YTPDMNREDV DYAIRKAFQV WSNVTPLKFS
160 170 180 190 200
KINTGMADIL VVFARGAHGD FHAFDGKGGI LAHAFGPGSG IGGDAHFDED
210 210 220 230 240
EFWTTHSGGT NLFLTAVHEI GHSLGLGHSS DPKAVMFPTY KYVDINTFRL
250
SADDIRGIQS LYG
Available mutants
F171D – mutant with improved stability – CAT #
G04MP12Cm
F171D, E219A – inactive mutant with improved stability – CAT #
G04MP12Ci
Purity
> 95% by SDS-PAGE. The protein is observed, in denaturing conditions, as a single band migrating at a molecular weight between 14.4 and 18.4 kDa.
Supplied as
0.2 mg/mL solution in Tris 20 mM pH 7.2, CaCl
2 10 mM, ZnCl
2 0.1 mM, NaCl 0.3 M, acetohydroxamic acid (AHA) 0.2 M. The concentration is calculated by the analysis of the absorbance at 280 nm, (ε
280 = 26930 M
-1cm
-1 calculated).
Specific activity
> 40 U/μg. Activity described as U=100 pmol/min at 25°C using a colorimetric assay with thiopeptide Ac-Pro-Leu-Gly-[2-mercapto-4-methyl-pentanoyl]-Leu-Gly-OC2H5 (Biomol) as substrate.
Storage
-80°C. After initial defrost, aliquot the product into individual tubes and refreeze at -80°C.
Avoid repeated freeze/thaw cycles.
Usage
Enzyme kinetic studies, cleavage of target substrates and screening of inhibitors.
RELEATED RESEARCH FIELDS
References
I. Bertini, et al. Proc Natl Acad Sci U S A. 2005 Apr 12; 102(15):5334-9.
S.D. Shapiro. Curr. Opin. Cell Biol. 1998, 10, 602.
S.D. Shapiro et al. J. Biol. Chem. 1993, 268, 23824.
A. Belaaouaj et al. J. Biol. Chem. 1995, 270, 14568.